What is three-phase emulsification technology?
New emulsification technology that requires no surfactant
“Three-phase emulsification method” is a new emulsification method that uses the physical action force (van der Waals attractive force) of the soft hydrophilic nano-particles instead of surfactants indispensable in conventional emulsification. This is a revolutionary method based on an entirely different approach from the conventional emulsification method that relies only on the size and shape of the particles and not on the unique properties of the substance. The technology can be used across a wide range of industrial fields to create emulsions that are adaptive to various environments.
Developer profile

Kazuo Tajima
Director and CTO (Chief Technology Officer)
Distinction Professor, Kanagawa University
Area of expertise: Colloid and surface chemistry
Message from the CTO
This technology is a new emulsification process based on hydrophilic nanoparticles, which is characterized by its uniqueness that, for example, it allows you to selectively make W/O- and O/W-type emulsions as you desire from the same emulsification particles. I hope that you will use this emulsion technology to help solve problems and develop new products.
Developer’s carrier
- February 9, 1938
- Born in Edogawa ward, Tokyo
- 1963
- Finished Master Course of Physical Chemistry,Graduate School of Science, Tokyo Metropolitan University (Master of Science)
- 1963
- Worked as Assistant at Faculty of Science, Tokyo Metropolitan University and in 1971, earned doctorate of Sciencedegree fromTokyo Metropolitan University.
- December 1970
- Receives the Matsunaga Fellowship Award (Study on Absorption of Surfactants Using Radiotracer Method)
- June 1975
- Receives the Itoh Fellowship Award(Study on Absorption of Surface-Active Substances to Phospholipids Membranes Using Radiotracer Method)
- From 1984
- 1984: Appointed as associate professor of facult of engineering at Kanagawa University; 1990: Professor.
Held various positions including chairman of the Agricultural / Applied Chemistry Subcommittee of the Science Council of Japan and chairman of the Japan Oil Chemists' Society. - February 1994
- Awarded the Excellent Papers award for Oil-and-Fat Technologies (Study on Vesicle Formation and Thermal Properties of Polyoxyethylene Hydrogenated Castor Oil-Based Nonionic Surfactants)
- March 2002
- Awarded the Award of the Japan Oil Chemists’ Society for his “Study on Self-Organizing Bodies Formed by Amphiphilic Agent”
- September 2002
- Awarded the Editor’s Award of Japan Oil Chemists’ Society(J. Oleo Science) (“Three-Phase Emulsion of Hexadecane with Dimyristoylphosphatidylglycerol Sodium Salt in Water: Interpretation by New Phase Transition in Bilayer Assembly”,J. Oleo Science,50,(No.6),475-484(2001))
Difference between Surfactant emulsification and the Three-phase emulsification
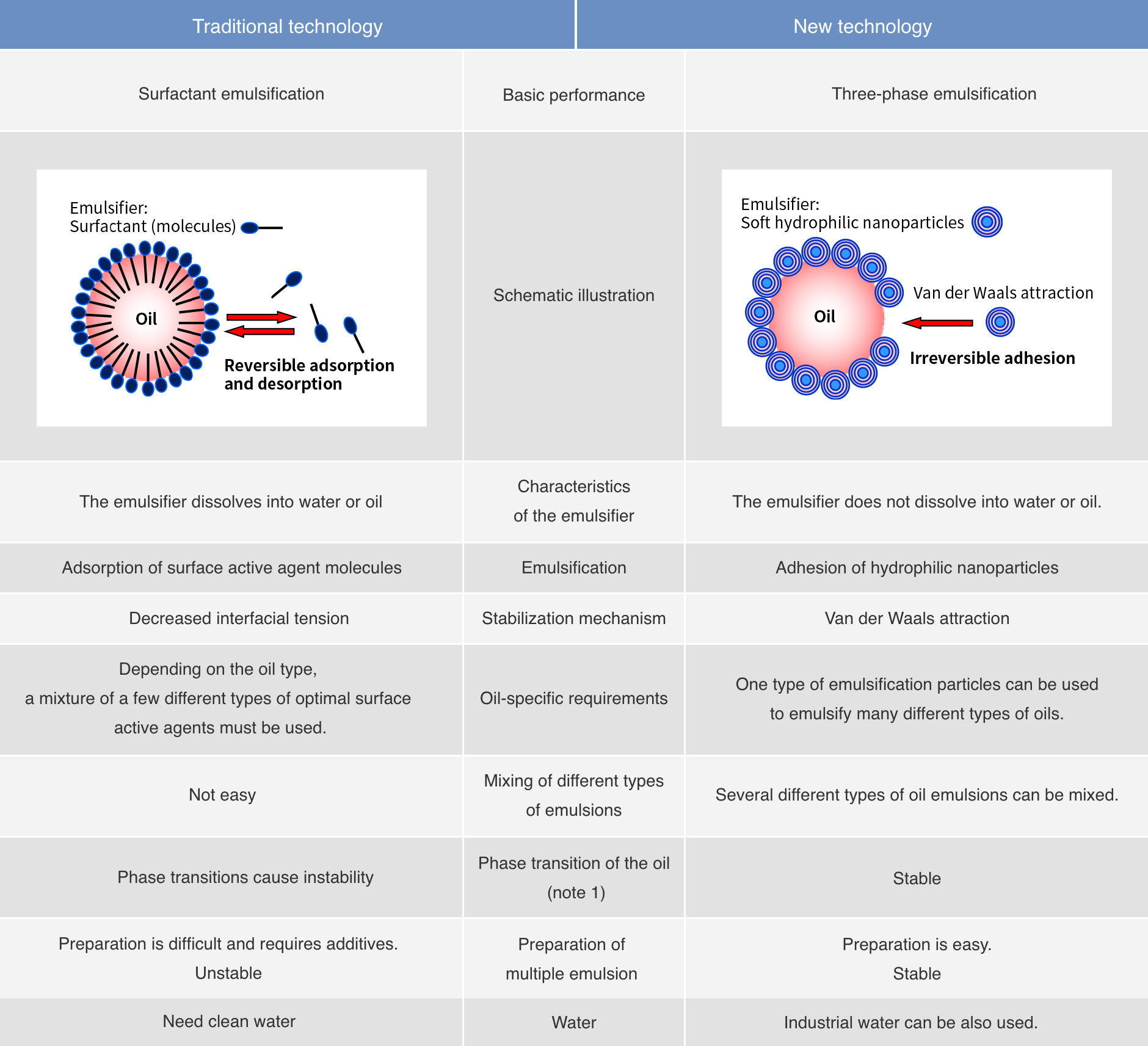
Note 1: Phase transition means that the structure of a substance (the form of the structure, i.e., gas, liquid, or solid form) is changed by external conditions to a different structure.
Characteristics of three-phase emulsification
- POINT1
- Decreased interfacial tension is not required
- POINT2
- Stable even when the emulsified oil droplet size is large
- POINT3
- Emulsions can be concentrated
- POINT4
- The oil phase is not separated even if the emulsion is diluted
- POINT5
- The emulsion is resistant to acids and salt
- POINT6
- Different types of oil emulsions can be mixed
- POINT7
- O/W type and W/O type emulsions can be made with the same composition
Unique physical properties provided by the three-phase emulsion structure
- POINT1
- Soft-capsulation and tekeout of emulsified droplets
- POINT2
- Solubilization
- POINT3
- Solid dispersion (to water or oil phase)
- POINT4
- Dual functions (adsorption and emulsification)
- POINT5
- Evaporation promotion (partial exposure and iceberg)
- POINT6
- Adhesion on the O/W and W/O interfaces


